Which regulations are applicable in UK?
The United Kingdom (UK) consists of two markets for medical devices: (1) The Great Britain market covering England, Scotland and Wales and (2) the Northern Ireland market. Different regulations apply in the two markets.
In Great Britain (GB) medical devices must comply with the UK Medical Device Regulations of 2002. At the time, UK MDR 2002 transposed the EU’s medical device Directives (AIMDD, MDD and IVDD) into UK law and this continues to be the legislation in force in Great Britain; now with GB specific requirements for UKCA marking and registration with MHRA. However, a 2023 amendment to UK MDR 2002 allows medical devices which comply with either the original EU medical device Directives, or with the latest EU medical device regulations (EUMDR/EUIVDR), to continue to be accepted on the GB market, still with some GB specific requirements.
Click the links for more details on the regulations that apply to medical devices in Great Britain (England, Scotland, Wales) and in Northern Ireland (NI), where medical devices must comply with the EU’s latest MDR or IVDR regulations.
Must I appoint a UK Responsible Person?
To place a device on the Great Britain market, any manufacturer based outside of the UK must appoint a UK Responsible Person (UK RP) established in the UK. See dedicated page.
Different requirements exist for the Northern Ireland market where EU authorised representatives are also recognised.
Must I register my device with MHRA?
In Great Britain all medical devices, including IVDs, custom-made devices and systems or procedure packs, must be registered with the MHRA before they can be placed on the market. In Northern Ireland the registration requirements depend on the location of the manufacturer or the Authorised Representative.
The MHRA only accepts registrations of devices from manufacturers or UK Responsible Persons that are based in the UK, or from Authorised Representatives based in Northern Ireland (for the purposes of the Northern Ireland market).
Click here to view the MHRA’s most recent guidance on registering medical devices for the markets in Great Britain and Northern Ireland.
What if I only intend my device to be used in Great Britian?
Manufacturers developing medical devices exclusively for the GB market can follow the UK's conformity assessment route to obtain UKCA marking. Click here for more information.
Click here for more information and which Approved Bodies can carry out assessments of medical devices on behalf of MHRA.
My device has a CE mark, is it still accepted in Great Britian?
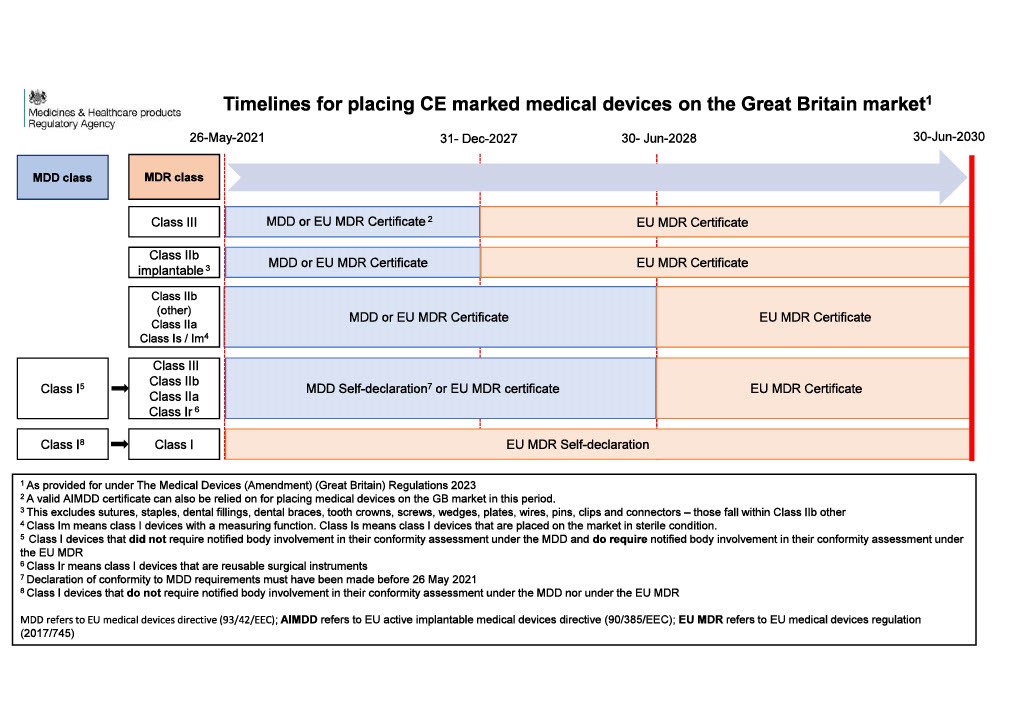
In 2023, the UK government extended the acceptance of CE marked medical devices on the Great Britain market. CE marked medical devices may continue to be placed on the Great Britain market in accordance with the timelines shown below.
Keep in mind, even with a CE mark, you must still appoint a UK respnsible Person (UKRP) to register the device with MHRA.