Ongoing recognition of CE marking in the GB market
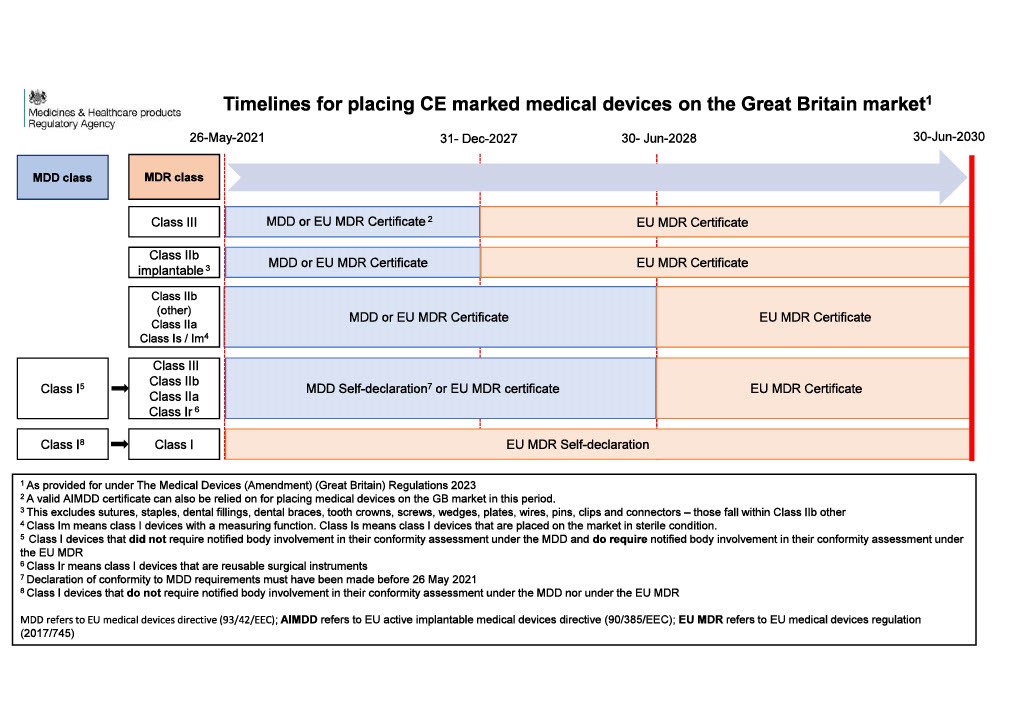
The UK government has put in place legislation (2023) to extend the acceptance of CE marked medical devices on the Great Britain market. The legislation provides that CE marked medical devices may be placed on the Great Britain market to the following timelines:
- general medical devices compliant with the EU medical devices directive (EU MDD) or EU active implantable medical devices directive (EU AIMDD) with a valid declaration and CE marking can be placed on the Great Britain market up until the sooner of expiry of certificate or 30 June 2028
- in vitro diagnostic medical devices (IVDs) compliant with the EU in vitro diagnostic medical devices directive (EU IVDD) can be placed on the Great Britain market up until the sooner of expiry of certificate or 30 June 2030, and
- general medical devices, including custom-made devices, compliant with the EU medical devices regulation (EU MDR) and IVDs compliant with the EU in vitro diagnostic medical devices regulation (EU IVDR) can be placed on the Great Britain market up until the 30 June 2030.
See info-graphic below
Appointing a UK Responsible Person
The post Brexit deadline for non-UK manufacturers to appoint a UK Responsible Person has now passed. To place a device on the Great Britain market, manufacturers based outside the UK must appoint a UK Responsible Person, established in the UK.
Different requirements exist for appointing a UK Responsible Person to place devices on the Northern Ireland market.
Registrations in Great Britain and Northern Ireland
All medical devices on the UK markets must now be registered with MHRA.
The post Brexit deadlines for registering devices on the Great Britain market have passed;
- By 1 May 2021: All active implantable medical devices, Class III medical devices, Class IIb implantable medical devices and IVD medical devices on List A.
- By 1 September 2021: All other Class IIb medical devices, Class IIa medical devices and IVD medical devices on List B and IVDs for self-testing.
- By 1 January 2022: Class I medical devices and all other IVDs.
The post Brexit deadlines for registering devices on the Northern Ireland market have passed;
- By 1 January 2021: Class I medical devices and all IVDs except those on Lists A or B or for self-testing.
- By 1 May 2021: All active implantable medical devices, Class III medical devices, Class IIb implantable medical devices and IVD medical devices on List A.
- By 1 September 2021: All other Class IIb medical devices, Class IIa medical devices and IVD medical devices on List B and IVDs for self-testing.
Click the link below to view the MHRA’s most recent guidance on registering medical devices for the markets in Great Britain and Northern Ireland.
You can view the MHRA's registration database using the link below.
Labelling requirements
Manufacturers of medical devices can use either the UKCA marking or the CE marking on devices they place on the Great Britain market.
Medical devices placed on the Great Britain market may have either a UKCA mark or a CE mark on the labelling, depending on which legislation the device has been certified under.
Follow the link below for more information about the UKCA marking. (note: different deadlines and rules may apply to marking of other types of products).